
Pardeep Kumar Bhardwaj
Pardeep Kumar Bhardwaj, Ph.D.
Scientist - F & Incharge, IBSD, Meghalaya Center, Shillong, Meghalaya
Academic Qualifications
• 2005-2011, Ph.D. (Biotechnology), CSIR-Institute of Himalayan Bioresource Technology, Palampur-176061 (Himachal Pradesh)/ Panjab University, Chandigarh-160014
• 1999-2001, M.Sc. (Biotechnology), Guru Jambheshwar University of Science & Technology, Hisar-125001 (Haryana)
Experience
• January 2025 – Till date, Scientist-F, Institute of Bioresources and Sustainable Development, Imphal-795001 (Manipur)
• February 2021 – December 2024, Scientist-E, Institute of Bioresources and Sustainable Development, Imphal-795001 (Manipur)
• August 2019 – February 2021, Scientist-E, Institute of Bioresources and Sustainable Development, Meghalaya Centre, Upper Shillong-793009 (Meghalaya)
• July 2018 - August 2019, Scientist-C, Institute of Bioresources and Sustainable Development, Meghalaya Centre, Upper Shillong-793009 (Meghalaya)
•
January 2011 - July, 2018, Scientist-C, Institute of Bioresources and
Sustainable Development, Sikkim Centre, Gangtok-737102 (Sikkim)
Awards & Fellowships
• 2023: Member of “Accreditation Panel of National Certification system for Tissue Culture Raised Plants” (NCSTCP) of Department of Biotechnology, Govt. of India, New Delhi.
• 2017: Certificate of outstanding contribution in reviewing “Industrial Crops and Products” an International journal, Elsevier.
• 2017: Best Poster Award for research work during “XIV Convention of BRSI, International Conference on Emerging Trends in Biotechnology for Waste Conservation 2017” at CSIR-NEERI, Nagpur (India).
• 2013: Felicitation by CSIR-IHBT, Palampur on National Science Day for research paper entitled “Braving the attitude of altitude: Caragana jubata at work in cold desert of Himalaya”.
• 2005: Awarded ‘Senior Research Fellowship’ by Council of Scientific and Industrial Research, Human Resource Development Ministry, Govt. of India.
Memberships (Professional Associations / Societies)
• Society For Ethnopharmacology, India (SFE/19/I-1573)
• The Society For Integrative Biosciences (LM008)
• DNA Society of India
• International Society of Chemical Ecology (Regular member)
• Biotech Research Society of India (1769).
• SBC, India (3287).
PhD’s Guided
• Bhusan Gurung (2019) Cloning and characterization of genes involved in biosynthesis of ginsenosides from Panax sokpayensis Shiva K. Sharma & Pandit. University of North Bengal.
Specialized Training
• Three months specialized training on medicinal plants certification at CSIR-Institute of Himalayan Bioresource Technology, Palampur (2009).
• Two days NABL training on “Laboratory quality & management system and Internal auditor training” based on ISO 19011-2002 at CSIR-Institute of Himalayan Bioresource Technology, Palampur (28-29th May, 2009).
• Two weeks training on “Gene Expression Analysis” at “CSIR-Institute of Himalayan Bioresource Technology”, Palampur (07-22nd November, 2011).
• Attended 4th Plant Proteomics Workshop entitled “Seabuckthorn- Genomics, Proteomics & Metabolomics” at Department of Botany, University of Delhi (23-27th December, 2019).
Other Achievements
• Involved in teaching Ph.D. course (Plant Molecular Biology- BT8004) at IBSD, Imphal
1. Research theme photo
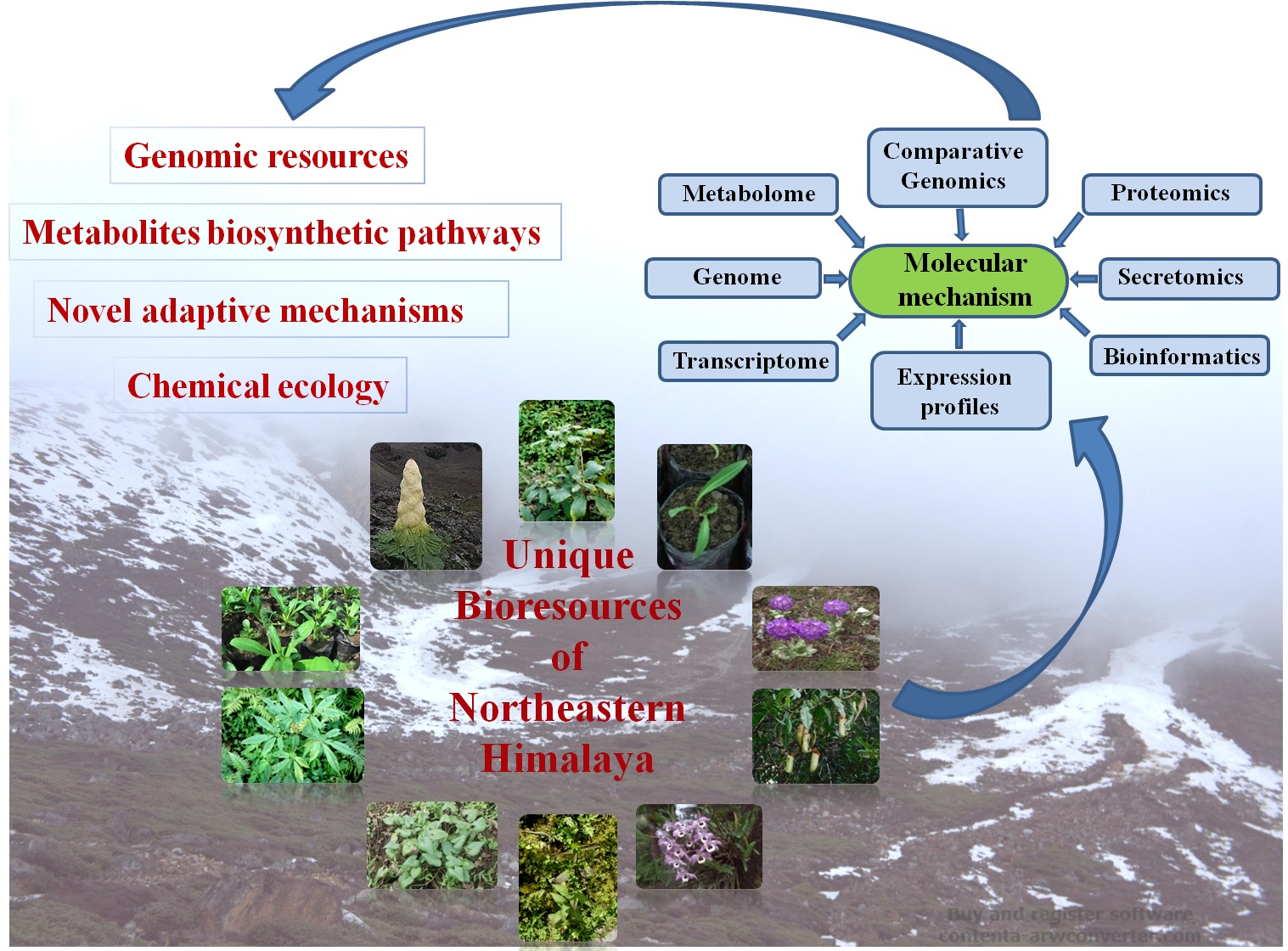
2. Verticals involved
Ethnobotany, ethnopharmacology and drug discovery
3. Research Area/Research Expertise
• Plant secondary metabolites and their biosynthetic pathways
• Metabolic engineering
• Molecular mechanisms of plant adaptation in high altitude
• Chemical ecology mainly secondary metabolites and endophytes
4. Research Summary
A. Current Research:
• Understanding the regulation of secondary metabolic pathways of important medicinal plants from Northeastern Himalaya.
Northeastern Himalaya is a globally significant region for ecosystems diversity and rich biodiversity. Due to its unique geographical dynamics, winds generated from the Bay of Bengal results in high rains in this region throughout the year. The climate and altitudinal variations of this region, along with varied ecological habitats have contributed to the development of rich vegetation with unique diversity of medicinal plants. Examples are Panax sokpayensis, Panax bipinnatifidus, Swertia chirayita, Picrorhiza scrophulariiflora, Paris polyphylla, Podophyllum hexandrum, Nepenthes khasiana, Rheum nobile etc. Realizing the uniqueness of niche environment, these medicinal plants offer an advantage in having much greater possibilities of novel biomolecules, genes, enzymes and biosynthetic pathways. Using metabolomics, transcriptomics and genomics approaches, we are trying to understand various metabolic pathways in these medicinal plants and characterization of regulatory genes for metabolic engineering.

Fig: Unique medicinal plants of Northeast Himalaya
• Understanding the Plant-Microbe interactions in medicinal plants of Northeastern Himalaya: from Chemical Ecology perspective.
Microbes and plants have a long lineage of association history which date back to ages from now. The association are crucial for establishment and maintenance of sustainable and stable ecosystem. Since plants are sessile and non-motile they constantly encounter biotic and abiotic stress. There is a constant tug of war between the plant host and the pathogen, the outcome is either resistance (symbiosis or mutualism) or disease (parasitism). Therefore, they have evolved overtime to help themselves survive in unfavourable conditions by associating themselves with beneficial microbes i.e. Endophytes, which promotes plant growth and development and helps in the production of vast array of metabolites which are beneficial to mankind. Recent focus has shifted on how these interaction and association occur and by what means they communicate to produce such effective metabolites. Here, Panax sokpayensis and Valeriana jatamansi are medicinal plants that have been targeted for the study. These are highly valued medicinal plants and the rhizome of these plants is harvested after 3 years to 5 years for commercial and domestic uses. Although several metabolites have been characterized from plants and microbes, the roles in between these partners are well not understood. It is known that plants select specific microbes in the soil, however no light is shed on how this selection specifically occurs. Plant-microbes communication begins with chemical signals through root exudates during colonization including expression of appropriate genetic machinery. Different plant species and varieties present different chemical characteristics and root exudation patterns after infection. Therefore, comparative transcriptome and metabolome analysis of endophyte infected and endophyte free plants will help us to understand the basis of crosstalk process involved in maintaining these plant-endophyte interactions.
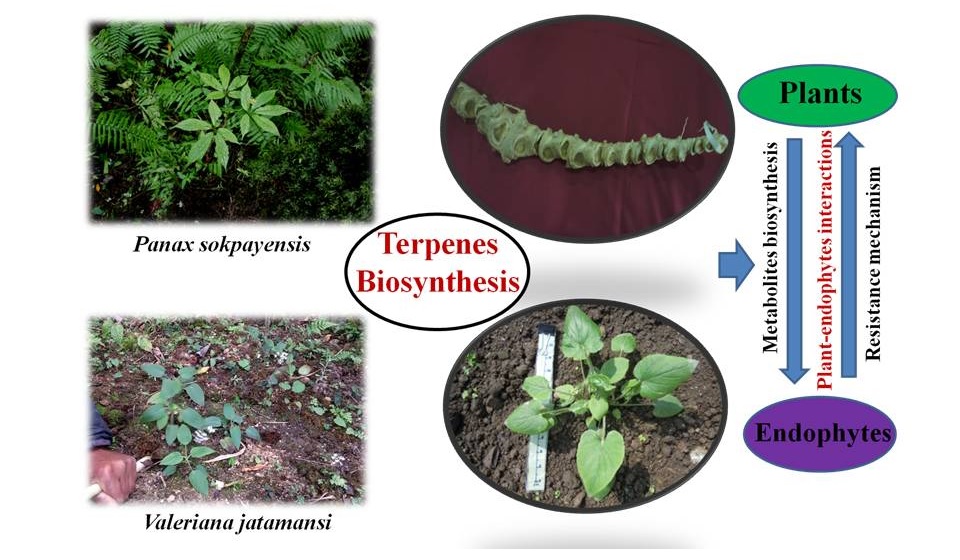
Fig: Plant-endophyte interactions and biosynthesis of secondary metabolites
B. Previous Notable Research:
1. Panax sokpayensis: A potential ginseng resource from Sikkim Himalaya
There are two Panax species namely Panax sokpayensis and Panax bipinnatifidus reported from this region which are naturally distributed along different elevations. Due to high medicinal value, roots/rhizomes of these species are harvested by locals for commercial and domestic use from wild habitats. Also, different ethnic groups are using these Panax species as traditional medicines. Therefore, it is important to scientifically validate the active ingredients in Panax sokpayensis and Panax bipinnatifidus and to find out elite species for commercial purpose. Research team at IBSD, quantified all the major ginsenosides (Rg1, Rg2, Rf, Re, Rd, Rb1 and Rb2) and confirmed their presence in 10 years old rhizomes of P. sokpayensis. The total contents of these major ginsenosides were found comparable with already reported total ginsenoside contents of Korean ginseng. Also, other parameters are compared for these major ginsenosides in Panax sokpayensis which are considered necessary for ginseng quality. The Rb1:Rg1 ratio in Panax sokpayensis (2.18) ranged between 1 and 3 which is considered as the characteristic of Asian ginseng (Korean and Chinese ginseng). Since ginsenoside contents present in Panax sokpayensis qualify all the parameters which are necessary for the quality of ginseng worldwide, it can be considered as a potential ginseng resource for large scale cultivation and commercialization.

Fig: Panax sokpayensis growing in forest, West Sikkim
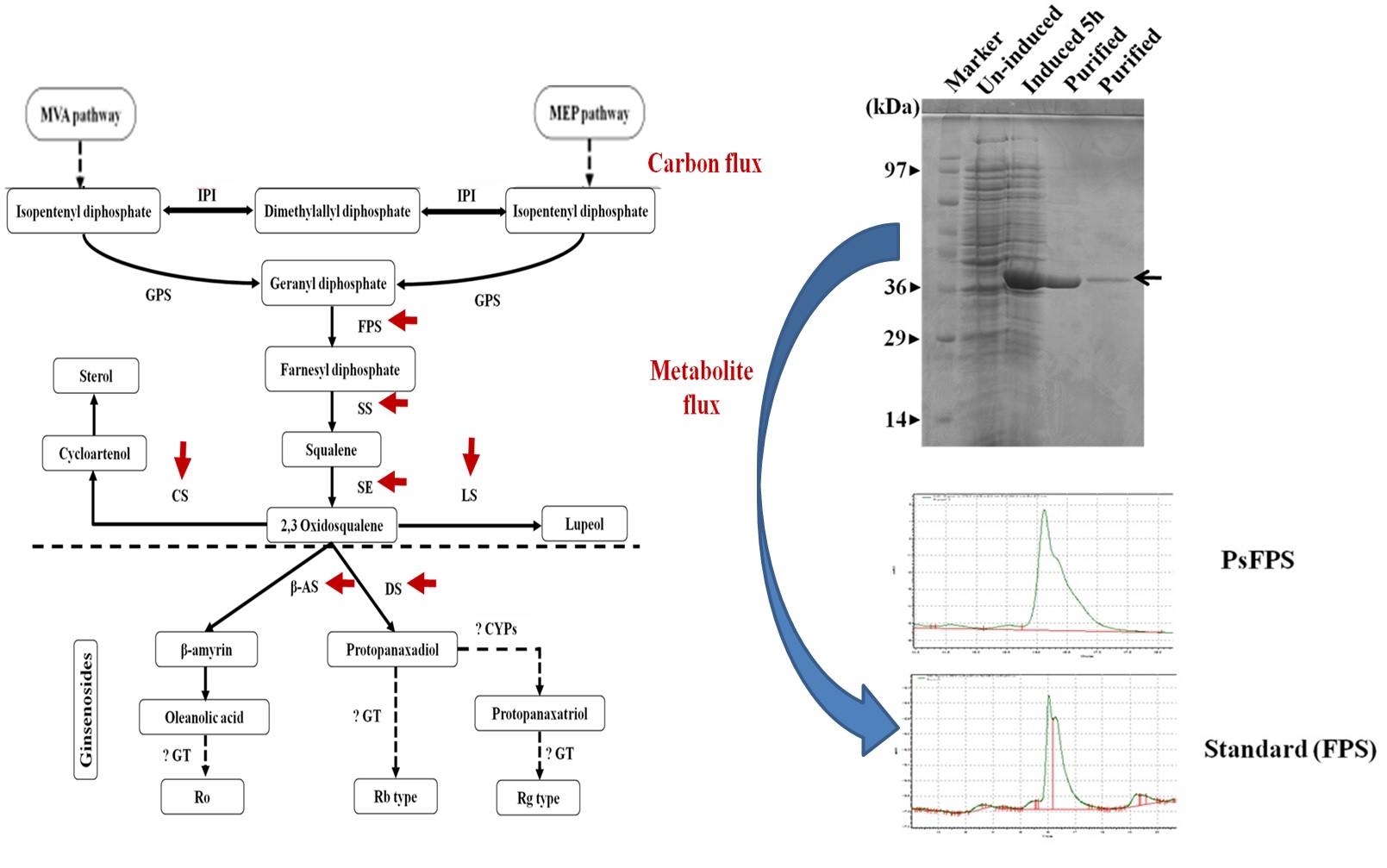
Fig: Characterization of key regulatory gene(s) involved in ginsenosides biosynthesis
A. Research Articles
1 Gurung P.D, Upadhyay A. K., Bhardwaj P.K., Sowdhamini R., Ramakrishnan U. (2019) Transcriptome analysis reveals plasticity in gene regulation due to environmental cues in Primula sikkimensis, a high altitude plant species. BMC Genomics, 20:989 (IF: 3.730): PMID: 31847812.
2 Gurung B., Bains S., Saha D., Singh K., Bhardwaj P.K.*, Sahoo D. (2019) Molecular cloning and characterization of farnesyl pyrophosphate synthase gene from Panax sokpayensis, a new Panax species from Sikkim Himalaya. Journal of Applied Research in Medicinal and Aromatic Plants, 14: 100215 (IF: 1.966): https://doi.org/10.1016/j.jarmap.2019.100215.
3 Gurung B., Bhardwaj P.K.*, Talukdar N.C. (2016) Subtractive transcriptome analysis of leaf and rhizome reveals differentially expressed transcripts in Panax sokpayensis. Functional & Integrative Genomics, 16: 619-639 (IF: 3.889): PMID: 27586658.
4 Gurung B., Bhardwaj P.K.*, Rai A., Sahoo D. (2018) Major ginsenoside contents in rhizomes of Panax sokpayensis and Panax bipinnatifidus. Natural Product Research, 32:2, 234-238. (IF: 1.928): https://doi.org/10.1080/14786419.2017.1343322.
5 Sathyanarayana N., Pittala R. K., Tripathi P. K., Chopra R., Singh H. R., Belamkar V., Bhardwaj P. K., Doyle J. J., Egan A. N. (2017) Transcriptomic resources for the medicinal legume Mucuna pruriens: de novo transcriptome assembly, annotation, identification and validation of EST-SSR markers. BMC Genomics, 18:409 (IF: 3.730): PMID: 28545396.
6 Shaw A.K., Bhardwaj P.K., Ghosh S., Ajhar I., Adhikari S., Adhikari A., Sherpa A.R., Saha S.K., Hossain Z. (2017) Profiling of BABA-induced differentially expressed genes of Zea mays using suppression subtractive hybridization. RSC Advances, 7, 43849. (IF: 2.936): https://doi.org/10.1039/C7RA06220F.
7 Rai A.K., Sanjukta S., Chaurasia R., Bhat I., Bhardwaj P.K., Sahoo D. (2017) Production of bioactive hydrolysate using protease, ?-glucosidase and ?-amylase of Bacillus spp. isolated from kinema. Bioresource Technology, 235:358-365 (IF: 5.807): PMID: 28384588.
8 Olsson S.B., Bhardwaj P.K., Brockmann A., Chandrashekara K., Gross J., Harari A., Hillier N.K, Joly E.J., Khesoh V., Lima E., Lofstedt C., PollF.M., Mir B.A., Raguso R.A., Rai S., Sarkar M.P., Seenivasangan T., Shankaar R.U. (2017) New Frontiers for Chemical Ecology: Reaffirming a Commitment to the Goteborg Resolution. Journal of Chemical Ecology, 43: 2-3 (IF: 2.419): PMID: 28185104.
9 Louis B., Waikhom S.D., Jose R.C., Goyari S., Bhardwaj P.K., Roy P., Talukdar N.C. (2017) Cochliobolus lunatus down regulates proteome at late stage of colonization and transiently alters StNPR1 expression in Solanum tuberosum L. Archives of Microbiology, 199(2):237-246. (IF: 1.607): DOI:10.1007/s00203-016-1297-2.
10 Kumar A., Sharma M., Bhardwaj P. K., Vats S. K., Singh D., Kumar S. (2016) Copper, zinc superoxide dismutase from Caragana jubata: A thermostable enzyme that functions under a broad pH and temperature window. Process Biochemistry, 51(10): 1434-1444. (IF: 2.616): https://doi.org/10.1016/j.procbio.2016.06.025
11 Shaw A.K., Hossain Z., Bhardwaj P.K., Sherpa A.R., Saha S.K. (2015) ?-aminobutyric acid mediated drought stress alleviation in maize (Zea mays L.) Environmental Science and Pollution Research, DOI:10.1007/s11356-015-5445-z (IF: 2.800): PMID: 26416125.
12 Bhardwaj P.K., Deep Mala, Kumar S. (2014) 2-Cys peroxiredoxin responds to low temperature and other external cues in Caragana jubata, a plant species of cold desert of Himalaya. Molecular Biology Reports, DOI 10.1007/s11033-014-3151-4. (IF: 1.889).
13 Louis B., Waikhom S.D., Roy P., Bhardwaj P.K., Singh W.M., Goyari S., Sharma C.K., Talukdar N.C. (2014) Secretome weaponries of Cochliobolus lunatus interacting with potato leaf at different temperature regimes reveal a CL[xxxx]LHM – motif. BMC Genomics, 15:213. (IF: 3.730): PMID: 24477582.
14 Louis B., Waikhom S.D., Roy P., Bhardwaj P.K., Singh W.M., Sharma C.K., Talukdar N.C. (2014) Invasion of Solanum tuberosum L. by Aspergillus terreus: A microscopic and proteomics insight on pathogenicity. BMC Research Notes, 7:350: PMID: 24917207.
15 Louis B., Waikhom S.D., Roy P., Bhardwaj P.K., Sharma C.K., Singh W.M., Talukdar N.C. (2014) Host range dynamics of Cochliobolus lunatus: From a biocontrol agent to a severe environmental threat. Biomed Research International, vol. 2014, Article ID 378372, PMID: 24987680 (IF: 2.583).
16 Bhardwaj P.K., Kapoor R., Deep Mala, Bhagwat G., Acharya V., Singh A.K., Vats S.K., Ahuja P.S., Kumar S. (2013) Braving the attitude of altitude: Caragana jubata at work in cold desert of Himalaya. Scientific Reports (Nature Publishing Group), 3:1022: DOI:10.1038/srep01022. (IF: 4.122): PMID: 23289064.
17 Waikhom S.D., Louis B., Roy P., Singh W.M., Bhardwaj P.K., Talukdar N.C. (2013) Scanning electron microscopy of pollen structure throws light on resolving Bambusa–Dendrocalamus complex: bamboo flowering evidence. Plant Systematics and Evolution, DOI 10.1007/s00606-013-0959-7. (IF: 1.452).
18 Bhardwaj P.K., Kaur J, Sobti R.C., Kumar S (2012) Identification and expression analysis of CjLTI, a novel low temperature responsive gene from Caragana jubata. Molecular Biology Reports, 39: 3197–3202. (IF: 1.889): PMID: 21701826.
19 Bhardwaj P.K., Kaur J, Sobti R.C., Ahuja P.S., Kumar S (2011) Lipoxygenase gene in Caragana jubata responds to low temperature, abscisic acid, methyl jasmonate and salicylic acid. Gene, 483: 49–53. (IF: 2.498): PMID: 21640803.
20 Ghawana S, Paul A, Kumar H, Kumar A, Bhardwaj P.K., Rani A, Singh R.S, Raizada J, Singh K and Kumar S (2011) An RNA isolation system for plant tissues rich in secondary metabolites. BMC Research Notes, 4: 85: PMID: 21443767.
21 Singh R.S., Gara R.K., Bhardwaj P.K., Malik S, Kumar R, Sharma M, Ahuja P.S., and Kumar S (2010) Expression of 3-hydroxy-3-methylglutaryl-CoA reductase, p-hydroxybenzoate-m-geranyltransferase and genes of phenylpropanoid pathway exhibits positive correlation with shikonins content in arnebia [Arnebia euchroma (Royle) Johnston]. BMC Molecular Biology, 11: 88. (IF: 2.795): PMID: 21092138.
22 Bhardwaj P.K., Ahuja P.S., Kumar S (2010) Characterization of gene expression of QM from Caragana jubata, a plant species that grows under extreme cold. Molecular Biology Reports, 37: 1003-1010. (IF: 1.889): PMID: 19757181.
23 Yogavel M, Mishra P.C., Gill J, Bhardwaj P.K., Dutt S, Kumar S, Ahuja P.S., Sharma A (2008) Structure of superoxide dismutase and implications for copper ion chelation. Acta Crystallographica Section D: Biological Crystallography D64: 892-901. (IF: 3.099): PMID: 18645238.
24 Singh K, Raizada J, Bhardwaj P.K., Ghawana S, Rani A, Singh H, Kaul K, Kumar S (2004) 26S rRNA-based internal control gene primer pair for reverse transcription-polymerase chain reaction-based quantitative expression studies in diverse plant species. Analytical Biochemistry, 335: 330–333. (IF: 2.275): PMID: 15556573.
B. Technology Transferred
1. Ghawana S., Singh K., Raizada J., Rani A., Bhardwaj P.K., Kumar S. A method for rapid isolation of RNA and a kit thereof. US Patent, WO 2007113614A1. The technology has been transferred to Bangalore Genei Pvt. Ltd. (As a member of CSIR-IHBT research team) http://geneilabs.com.bh-in-14.webhostbox.net/wp/product/raflex-total-rna-isolation-kit-50-preps/
C. Patents
1. Bhardwaj P.K., Kumar A., Kishor A., Ghawana S., Rani A., Singh K., Singh H., Singh R.S., Kumar H., Sood P., Dutt S., Kumar S., Ahuja P.S. (2017) Method of cloning stable stress tolerant superoxide dismutase using universal primers. EP-2268661-B1 (Granted).
2. Bhardwaj P.K., Kumar A., Kishor A., Ghawana S., Rani A., Singh K., Singh H., Singh R.S., Kumar H., Sood P., Dutt S., Kumar S., Ahuja P.S. (2015) Method of cloning stable stress tolerant superoxide dismutase using universal primers. US 9212350 B2 (Granted).
3. Ghawana S., Singh K., Raizada J., Rani A., Bhardwaj P.K., Kumar S. (2014) A method for rapid isolation of RNA and a kit thereof. IN 259562 (Granted).
4. Bhardwaj P. K., Kapoor R., Bhagwat G., Ahuja P. S., Kumar S. (2014) DNA sequence in plant Caragana jubata with freeze tolerance. US Patent, US 2014/0018526 A1 (Filed).
5. Bhardwaj, P.K., Sahoo, R., Kumar, S., and Ahuja, P.S. (2013) Superoxide dismutase gene from Potentilla atrosanguinea and its expression in heterologous system. AU 2006341292 (Granted).
6. Ghawana S., Singh K., Raizada J., Rani A., Bhardwaj P.K., Kumar S. (2013) A method for rapid isolation of RNA and a kit thereof. EP2004822 (Granted).
7. Ghawana S., Singh K., Raizada J., Rani A., Bhardwaj P.K., Kumar S. (2012) A method for rapid isolation of RNA and a kit thereof. CN ZL20068005561.3 (Granted).
8. Ghawana S., Singh K., Raizada J., Rani A., Bhardwaj P.K., Kumar S. (2012) A method for rapid isolation of RNA and a kit thereof. AU 2006341291 (Granted).
9. Bhardwaj, P.K., Sahoo, R., Kumar, S., and Ahuja, P.S. (2011) A gene encoding autoclavable superoxide dismutase and its expression in E. coli. US patent 7,888,088 (Granted).
10. Bhardwaj P.K., Kumar A., Kishor A., Ghawana S., Rani A., Singh K., Singh H., Singh R.S., Kumar H., Sood P., Dutt S., Kumar S., Ahuja P.S. (2008) A method for cloning functional gene of copper/zinc superoxide dismutases using oligonucleotide primers. IN 0035nf2008. (Filed).
11. Ghawana S., Singh K., Raizada J., Rani A., Bhardwaj P.K., Kumar S. (2007) A method for rapid isolation of RNA and a kit thereof. US Patent, WO 2007113614A1 (Granted).
Group Members (Details):
|
1. |
Ms. Shweta Rai, JRF, Chemical Ecology Email: shwetabtwarai@gmail.com Research Topic: Understanding the molecular dynamics of plant-endophyte interactions in Panax sokpayensis . Expected outcomes: Comparative transcriptome and metabolome analysis of endophyte infected and endophyte free plants will help us to understand the basis of crosstalk process involved in maintaining these plant-endophyte interactions. |
|
|
2. |
Mr. Shashi Kumar Yadav, JRF, IBSD Core Email: yadavkumarshashi@gmail.com Research Topic: Metabolomics and transcriptome analysis of Panax wangianus , a Panax species from Meghalaya. Expected Outcome: This work will decipher the ginsenoside contents and genomic information in this Panax species from Northeastern region. |
|
|
Previous Members (Alumni) |
||
|
1. |
Dr Bhusan Gurung, Project Assistant, IBSD Core Email: bhusan27@hotmail.com Worked on – Cloning and characterization of genes involved in biosynthesis of ginsenosides from Panax sokpayensis Shiva K. Sharma & Pandit. Currently working at: Research Associate, Sikkim State Council of Science & Technology, Gangtok, Sikkim |
|
|
2. |
Ms Ophilia Ibapalei Lyngdoh Mawphlang, JRF, IBSD Core Email: ophiliaibapalei12@gmail.com Worked on – Functional annotation of secoiridoids/xenthones biosynthetic pathways genes in leaf and root tissue of Swertia chirayta . Currently working at: State Education Department, Meghalaya |
|
A. Core/Internal
a. Present:
|
Sl. No. |
Duration (From-To) |
Title of Project/ Grant |
PI/Co-PI |
Funding Agency |
|
|
1 |
September 2011-Continue |
Understanding the regulation of secondary metabolic pathways of important medicinal plants from North East Region of India. |
PI |
IBSD |
|
b. Past: N/A
|
Sl. No. |
Duration (From-To) |
Title of Project/ Grant |
PI/Co-PI |
Funding Agency |
|
|
|
|
|
|
IBSD |
|
B. Extramural:
a. Present:
|
Sl. No. |
Duration (From-To) |
Title of Project/ Grant |
PI/Co-PI |
Funding Agency |
|
|
1 |
April 2015-Continue |
Chemical Ecology of the North East Region of India: A Collaborative Programme Linking NER and Bangalore Researcher. |
Co-PI |
DBT |
|
b. Past: N/A
|
Sl. No. |
Duration (From-To) |
Title of Project/ Grant |
PI/Co-PI |
Funding Agency |
Amount |
|
|
|
|
|
|
|




